This website uses cookies to enhance your browsing experience. By continuing to browse this website, you agree to accept our use of cookies. For more information, please review our Privacy Policy.
How to use ADLARITY®
Once-weekly treatment for dementia associated with Alzheimer’s disease
Plan. Peel. Place.
Select a step below to see more.
Plan
Before applying ADLARITY
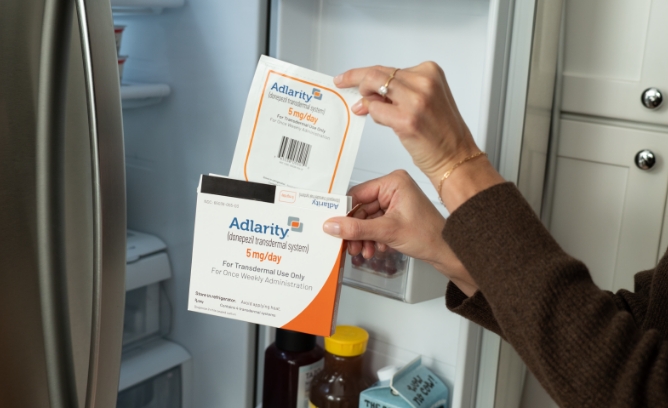
- ADLARITY must be kept in the refrigerator. When you’re ready to apply it, take out 1 pouch

- Before applying ADLARITY to the skin, allow it to reach room temperature
- Use ADLARITY within 24 hours of taking the pouch out of the refrigerator
- Do not use external heat sources to warm ADLARITY
Keeping track
- Apply ADLARITY on the same day and at the same time each week—be sure to change the skin area you choose
- Whenever you remove a used ADLARITY transdermal system, you must wait a full 14 days before using the same area again

Ask your doctor about the ADLARITY Weekly Planner
Ask your doctor about the ADLARITY Weekly Planner
Keep in mind
- Do not use ADLARITY if it becomes damaged in the application process or if it has expired. Call your pharmacy or your doctor
- Always apply ADLARITY to intact skin immediately after removal from the pouch. Never apply it over folds of skin or across the spine
Peel
How to remove Liner 1
- There is a 2-piece liner on the back of ADLARITY. You will need to remove each piece separately
- Liner 1 is the shorter side of the liner
- Always wash your hands before and after handling ADLARITY
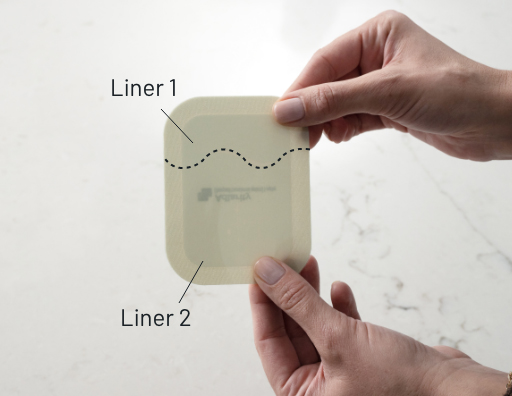
- To remove Liner 1, hold ADLARITY with the liner facing you

- Bend it at the wavy cut in the middle (this will separate the liners)

- Remove Liner 1 only. The sticky side of ADLARITY is now exposed—be careful not to touch it
Place
How to remove Liner 2 and place ADLARITY on the skin
- Place the exposed side of ADLARITY against the skin. Once it is on the skin, you can begin to remove Liner 2
- Liner 2 is the longer side of the liner
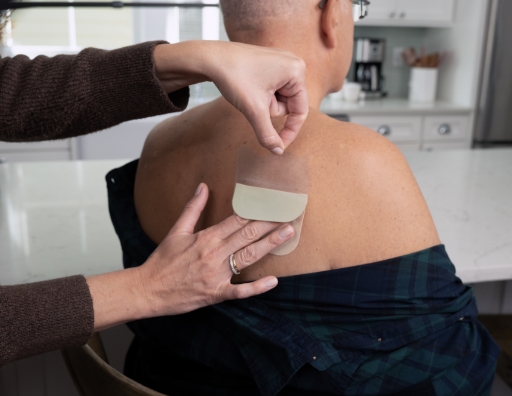
- Slowly peel away Liner 2 with 1 hand, while gently smoothing ADLARITY into place with the other
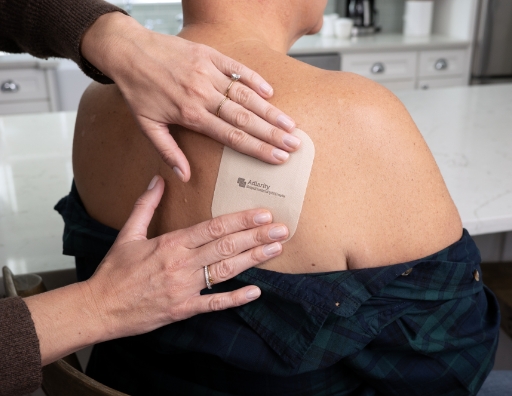
- Smooth it out with your fingers
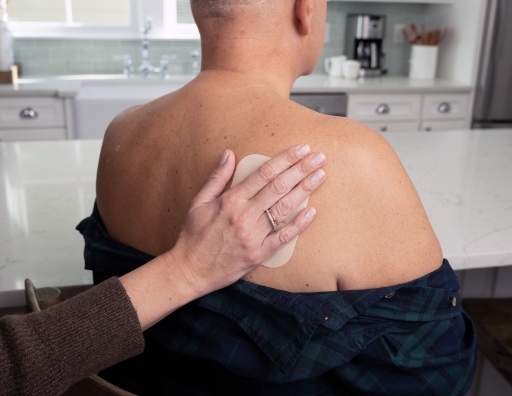
- Press firmly for 30 seconds to make sure that the edges stick to the skin
Please read the complete Instructions for Use before using ADLARITY.
Wearing ADLARITY
- The recommended application site is the back, but there are 8 different skin areas where you can choose to apply ADLARITY
- You’ll need to change the skin area each week
- ADLARITY can be applied horizontally or vertically
- Only 1 ADLARITY transdermal system should be worn at a time
Recommended areas to apply
If you are a caregiver applying ADLARITY:

If you are a patient who is self-applying:

Removing ADLARITY
After 7 days have passed, it is time to apply a new ADLARITY to a different location. First, remove the one that was previously applied.
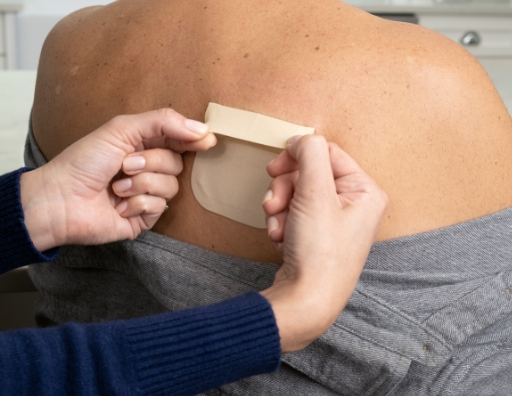
- Use both thumbs

- Slowly and evenly peel ADLARITY off the skin, from top to bottom
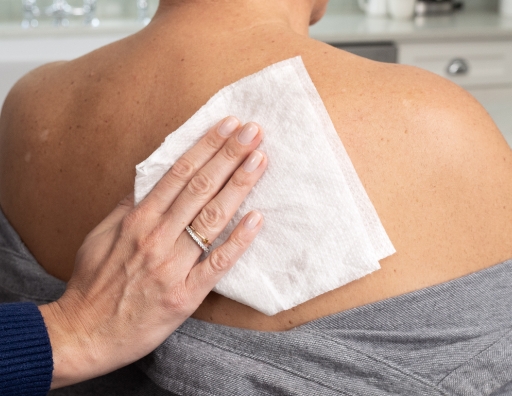
- Make sure no pieces are left on the skin. If there are, remove them with your fingers; if needed, ONLY use mineral or baby oil
Disposing of ADLARITY
It is important to properly dispose of ADLARITY because there may still be medicine in it after the 7-day wear period is over.
- Fold ADLARITY in half (the sticky sides should stick together)
- Throw it away in the household trash ONLY

Always take note of…
Where you place ADLARITY
- Each ADLARITY prescription comes with 4 transdermal systems—enough for 4 weeks of treatment. It’s helpful to choose your skin areas for each week once you fill your prescription
- Consider selecting at least 3 skin areas with each prescription
When you change ADLARITY
- ADLARITY is worn continuously for 7 days
- Apply ADLARITY on the same day and at the same time each week
The 2-week rule Every time you remove ADLARITY, you must wait a full 14 days before using the same skin area again. This will lower the chance of skin irritation.
What is ADLARITY used for?
IMPORTANT SAFETY INFORMATION
Who should not take ADLARITY?
Do not take ADLARITY if you:
- Are allergic to donepezil, certain medications called piperidine derivatives, or any of the ingredients in ADLARITY
What is ADLARITY used for?
IMPORTANT SAFETY INFORMATION
Who should not take ADLARITY?
Do not take ADLARITY if you:
- Are allergic to donepezil, certain medications called piperidine derivatives, or any of the ingredients in ADLARITY
- Have a history of a skin reaction called allergic contact dermatitis to ADLARITY
What Warnings should I know about ADLARITY?
- Some people experienced skin reactions that include redness and itching at the application site when using ADLARITY. Stop using ADLARITY and call your healthcare provider if you experience skin reactions that do not improve within 48 hours after the transdermal system is removed. Skin reactions include increased redness or swelling, peeling or blistering of the skin, or spreading beyond the application site.
- The class of medicine that includes ADLARITY may cause slow heartbeat and fainting. Call your doctor right away if you feel faint or lightheaded while using ADLARITY.
- Donepezil, the active ingredient in ADLARITY, may cause diarrhea, nausea, and vomiting. In most cases these effects have been transient, although some cases lasted 1 to 3 weeks.
- The class of medicine that includes ADLARITY may cause more stomach acid. This increases the chance of ulcers and bleeding. The risk is higher for people who have had ulcers or take NSAIDs. Call your healthcare provider right away if you have heartburn or stomach pain that is new or does not go away, blood in your vomit, dark vomit that looks like coffee grounds, or bowel movements or stools that look like black tar.
- Although not observed in clinical trials of ADLARITY, problems passing urine may occur.
- The class of medicine that includes ADLARITY are believed to have some potential to cause seizures. However, seizures may also be caused by Alzheimer’s disease.
- The class of medicine that includes ADLARITY may cause worsening of lung problems in people with asthma or other lung disease.
What should I tell my health care provider?
Tell your doctor about all of your present or past health conditions, including:
- Any heart problems, including problems with irregular, slow, or fast heartbeats
- Stomach ulcers
- Problems passing urine
- Seizures
- Asthma or lung problems
- Pregnant or plan to become pregnant. It is not known if ADLARITY can harm an unborn baby
- Breast-feeding. It is not known if donepezil passes into breast milk
- A previous skin reaction to the ADLARITY patch
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal products. ADLARITY and other medicines may affect each other when used at the same time. Especially tell your healthcare provider if you take medicines called nonsteroidal anti-inflammatory drugs (NSAIDs).
Tell your healthcare provider or dentist that you use the ADLARITY transdermal system before you have surgery, medical procedures, or dental surgery or procedures.
What are the side effects of ADLARITY?
The most common side effects of ADLARITY (>3%) were headache (15%), application site itching (9%), muscle spasms (9%), sleeplessness (7%), abdominal pain (5%), application site skin irritation (6%), constipation (5%), diarrhea (4%), application site pain (4%), dizziness (4%), abnormal dreams (4%), and skin tearing (4%).
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/MedWatch or call 1‑800‑FDA‑1088.
Please click here for Full Prescribing Information and Patient Information.
