This website uses cookies to enhance your browsing experience. By continuing to browse this website, you agree to accept our use of cookies. For more information, please review our Privacy Policy.
A once-weekly
treatment for
Alzheimer’s dementia
ADLARITY® is the first and only FDA-approved once-weekly transdermal system that consistently delivers donepezil while it's worn.
Not actual size.
ADLARITY® is the first and only FDA-approved once-weekly transdermal system that consistently delivers donepezil while it's worn.
Not actual size.

ADLARITY is not a daily pill. It is a once-weekly donepezil transdermal system.
Discover ADLARITY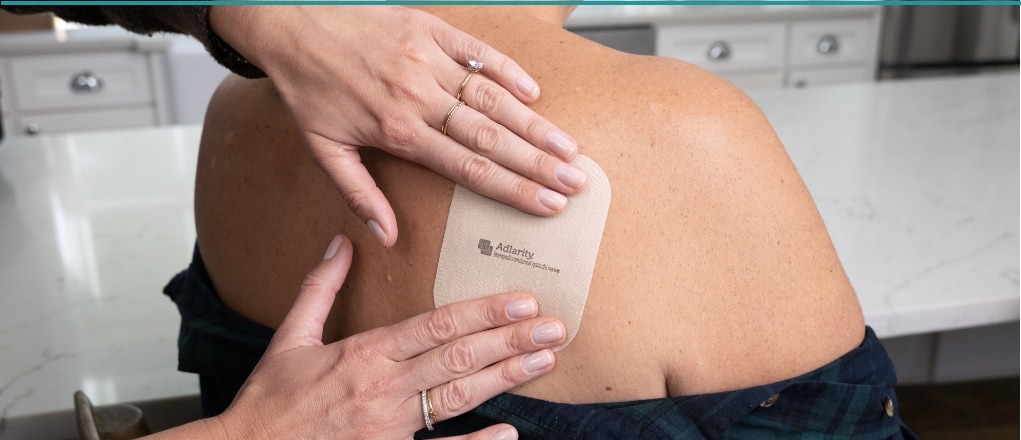
ADLARITY is applied to the skin. See how it is done.
Learn more
Learn about access and coverage for ADLARITY.
Explore optionsHere is a helpful video to get started with ADLARITY
What is ADLARITY used for?
IMPORTANT SAFETY INFORMATION
Who should not take ADLARITY?
Do not take ADLARITY if you:
- Are allergic to donepezil, certain medications called piperidine derivatives, or any of the ingredients in ADLARITY
What is ADLARITY used for?
IMPORTANT SAFETY INFORMATION
Who should not take ADLARITY?
Do not take ADLARITY if you:
- Are allergic to donepezil, certain medications called piperidine derivatives, or any of the ingredients in ADLARITY
- Have a history of a skin reaction called allergic contact dermatitis to ADLARITY
What Warnings should I know about ADLARITY?
- Some people experienced skin reactions that include redness and itching at the application site when using ADLARITY. Stop using ADLARITY and call your healthcare provider if you experience skin reactions that do not improve within 48 hours after the transdermal system is removed. Skin reactions include increased redness or swelling, peeling or blistering of the skin, or spreading beyond the application site.
- The class of medicine that includes ADLARITY may cause slow heartbeat and fainting. Call your doctor right away if you feel faint or lightheaded while using ADLARITY.
- Donepezil, the active ingredient in ADLARITY, may cause diarrhea, nausea, and vomiting. In most cases these effects have been transient, although some cases lasted 1 to 3 weeks.
- The class of medicine that includes ADLARITY may cause more stomach acid. This increases the chance of ulcers and bleeding. The risk is higher for people who have had ulcers or take NSAIDs. Call your healthcare provider right away if you have heartburn or stomach pain that is new or does not go away, blood in your vomit, dark vomit that looks like coffee grounds, or bowel movements or stools that look like black tar.
- Although not observed in clinical trials of ADLARITY, problems passing urine may occur.
- The class of medicine that includes ADLARITY are believed to have some potential to cause seizures. However, seizures may also be caused by Alzheimer’s disease.
- The class of medicine that includes ADLARITY may cause worsening of lung problems in people with asthma or other lung disease.
What should I tell my health care provider?
Tell your doctor about all of your present or past health conditions, including:
- Any heart problems, including problems with irregular, slow, or fast heartbeats
- Stomach ulcers
- Problems passing urine
- Seizures
- Asthma or lung problems
- Pregnant or plan to become pregnant. It is not known if ADLARITY can harm an unborn baby
- Breast-feeding. It is not known if donepezil passes into breast milk
- A previous skin reaction to the ADLARITY patch
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal products. ADLARITY and other medicines may affect each other when used at the same time. Especially tell your healthcare provider if you take medicines called nonsteroidal anti-inflammatory drugs (NSAIDs).
Tell your healthcare provider or dentist that you use the ADLARITY transdermal system before you have surgery, medical procedures, or dental surgery or procedures.
What are the side effects of ADLARITY?
The most common side effects of ADLARITY (>3%) were headache (15%), application site itching (9%), muscle spasms (9%), sleeplessness (7%), abdominal pain (5%), application site skin irritation (6%), constipation (5%), diarrhea (4%), application site pain (4%), dizziness (4%), abnormal dreams (4%), and skin tearing (4%).
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/MedWatch or call 1‑800‑FDA‑1088.
Please click here for Full Prescribing Information and Patient Information.
